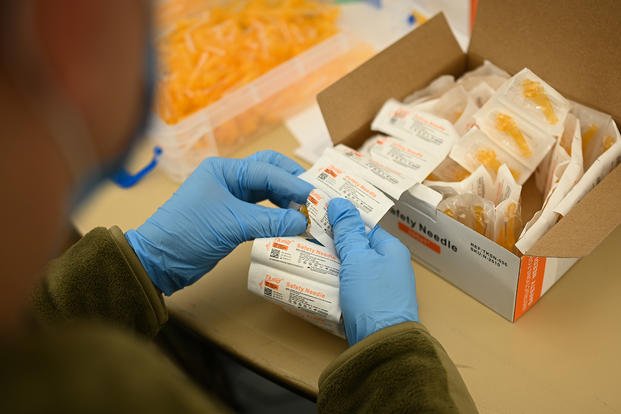
Two U.S. service members who have recovered from COVID-19 are asking a federal judge to put an immediate stop to the Defense Department’s mandatory COVID-19 vaccine order.
Army Staff Sgt. Daniel Robert and Marine Corps Staff Sgt. Hollie Mulvihill filed a suit Aug. 17 in the U.S. District Court for the District of Colorado seeking an exception to the order for military members who have recovered from the illness.
But last week, the pair’s attorneys stepped up the effort, requesting a temporary injunction to stop all vaccinations and a judge’s order that would require the DoD to exempt everyone with natural immunity from the mandate.
“Service members that have natural immunity, developed from surviving the virus, should be granted a medical exception from compulsory vaccination because the DoD instruction policy reflects the well-established understanding that prior infection provides the immune system’s best possible response to the virus,” the lawsuit states.
The Centers for Disease Control and Prevention recommends the COVID-19 vaccine for patients who have contracted COVID-19. A study published Aug. 6 by the CDC found that people in Kentucky who had COVID-19 and were unvaccinated were twice as likely to be reinfected than those with COVID-19 who were fully vaccinated.
“These data further indicate that COVID-19 vaccines offer better protection than natural immunity alone and that vaccines, even after prior infection, help prevent reinfections,” according to the study.
But Todd Callender, a representative for the plaintiffs and an attorney with Disabled Rights Advocates, argues that his clients and military personnel who have had COVID-19 are relatively young and healthy and therefore should be allowed to make decisions about vaccination on their own.
“These are the healthiest people on the planet in their age group … so why are we rushing? What is the compelling reason forcing the military to say, ‘You must take this vaccine, regardless of what the law says?'” Callender said.
The COVID-19 coronavirus is transmitted from one person to another via airborne droplets emitted while breathing, speaking, coughing or sneezing, and even individuals who don’t have symptoms can spread the virus, posing a risk to others and perpetuating transmission — and the pandemic. CDC officials repeatedly have pushed for vaccination even among the young and healthy to avoid continued transmission, which also leads to increased risk of new variants emerging.
Robert is an infantryman currently stationed at Fort Bragg, North Carolina, while Mulvihill is an air traffic controller assigned to Marine Corps Air Station New River, also in North Carolina, according to court documents.
The two are asking that their suit be certified as a class action to represent an unknown percentage of the nearly 250,000 troops who have contracted COVID-19 and don’t think they need vaccination.
In their case, they argue that Army Regulation 40-562 gives troops who are documented survivors of the infection a medical exemption from vaccination because of acquired immunity from having had an illness.
According to the regulation, “General examples of medical exemptions include the following — underlying health conditions … evidence of immunity based on serologic tests, documented infection or similar circumstances.”
As part of their suit, the plaintiffs also are pointing to the fact that some of the vaccine stock now being used by the DoD was manufactured and issued before the Pfizer-BioNTech vaccine received full FDA approval on Aug. 23. Existing legal precedent suggests that vaccines must receive full approval before troops could be required to receive them.
“The law says they don’t have to have [a vaccine]. We just want [them] to follow [their] own law,” Callender said during an interview with Military.com.
According to the Food and Drug Administration, the FDA-approved vaccine, licensed under the brand name Comirnaty, is exactly the same as the Pfizer-BioNTech vaccine tested in clinical trials and authorized for emergency use, or EUA. The FDA says they “can be used interchangeably to provide the COVID-19 vaccination series without presenting any safety or effectiveness concerns.”

 320 views today
320 views today  02:28 EST
02:28 EST